
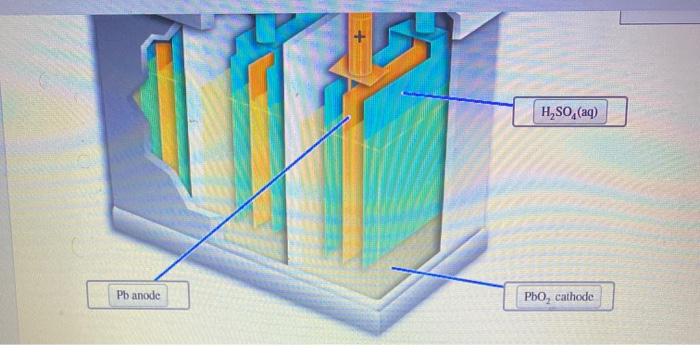
They can also improve the electric performance. Accordingly small quantities of other metals are added to the lead to provide the additional strength required. These constituents are all contained within a plastic container which acts to keep the electrolyte in and the battery together.Īlthough the plates or electrodes of the lead acid battery are designated as being lead, lead itself is too soft to be used on its own.

Lead acid battery basics: how do they work

The gases produced inside VRLA battery were made to recombine inside and the battery was sealed for the production of batteries used for standard operating conditions. These valve-regulated lead-acid batteries, VRLA by utilising an oxygen recombination mechanism. This was overcome by inserting a valve into the battery. Once the basic lead acid battery technology was established, the next major development involved addressing the problem that existed of water loss and the electrolyte drying up.īatteries needed period topping up with distilled water in order to ensure their operation. This speeded up the forming process considerably as the negative plate became lead only, and the positive plate oxidised to become lead peroxide. He managed to make the forming process much shorter by using some strips of lead oxide onto the plates. Plante's basic cell was later improved by a further French engineer named Faure. The battery then increased its capacity over successive charge discharge cycles. The battery then had to be 'formed' by charging it so that one of the plates oxidised. The first lead acid cells were made from lead plates. The idea was originally proposed by a French Physicist named Gaston Plante in 1860.Īlthough another French scientist named Gautherot had discovered that platinum or silver wires that had been used to electrolyse saline water produced a current for a short duration, this was never developed into a workable battery. The lead acid battery was the first form of rechargeable battery to be developed. Typical lead acid battery in a car Lead acid battery history These new lithium ion EV batteries are able to provide the power to drive the vehicle as well as providing the power for the electronic and electrical equipment within the car as well.Īccordingly the use of lead acid battery technology is reducing as the EV batteries are used increasingly for newer cards and other vehicles. However, lead acid battery technology is now being supplanted by lithium ion battery technology. The lead acid battery has many advantages for automotive and many other uses: they have a large current and surge capability, which is ideal when being used to start internal combustion engines.Īs a technology, lead acid batteries are well a well established technology and they can be easily manufactured with relatively low technology equipment. They are probably best known for their use in conventional internal combustion automotive vehicles where they supply the power for everything from starting, to the electronics and much more.
Lead acid batteries are cheap, convenient and they work for many battery power applications.
#ANODE AND CATHODE IN LEAD ACID BATTERY SERIES#
How Do Lead Acid Batteries Work What are lead acid batteries and how do lead acid batteries work - a look at the technology, operation, advantages and the construction of the lead acid battery.īattery technology overview Battery definitions & terms Battery capacity & life Batteries / cells in series & parallel Zinc carbon Alkaline cells Zinc air cells Lithium primary battery NiCad NiMH Li-ion Lead acid


 0 kommentar(er)
0 kommentar(er)
